Hypofibrinogenemia Market Set to Grow as New Treatments Emerge During the Forecast Period (2025–2034) | DelveInsight
DelveInsight projects a promising growth trajectory for the hypofibrinogenemia market across the 7MM from 2025 to 2034. This anticipated growth is primarily driven by the forthcoming introduction of emerging therapies and a rise in the number of Hypofibrinogenemia cases.
New York, USA, May 22, 2025 (GLOBE NEWSWIRE) -- Hypofibrinogenemia Market Set to Grow as New Treatments Emerge During the Forecast Period (2025–2034) | DelveInsight
DelveInsight projects a promising growth trajectory for the hypofibrinogenemia market across the 7MM from 2024 to 2034. This anticipated growth is primarily driven by the forthcoming introduction of emerging therapies and a rise in the number of Hypofibrinogenemia cases.
DelveInsight’s Hypofibrinogenemia Market Insights report includes a comprehensive understanding of current treatment practices, emerging hypofibrinogenemia drugs, market share of individual therapies, and current and forecasted hypofibrinogenemia market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Hypofibrinogenemia Market Report
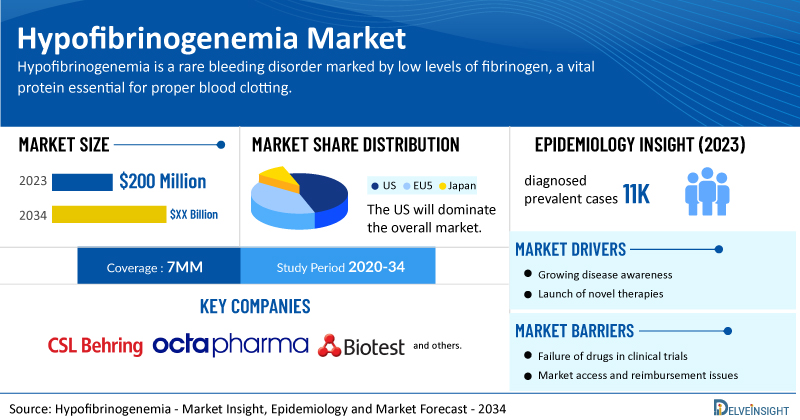
- According to DelveInsight’s analysis, the market size of hypofibrinogenemia in the 7MM was approximately USD 200 million in 2023 and is projected to increase during the forecast period (2025–2034).
- In the US, cryoprecipitate generated the highest revenue of ~USD 70 million for congenital hypofibrinogenemia in 2023.
- According to DelveInsight, the total diagnosed prevalent cases of hypofibrinogenemia in the 7MM were found to be nearly 11K in 2023.
- Prominent companies working in the domain of hypofibrinogenemia include CSL Behring, Octapharma, Biotest AG, and others.
- Some of the key hypofibrinogenemia treatments include RiaSTAP, Fibryna, AdFIrst (BT-524), and others.
Discover which therapies are expected to grab the hypofibrinogenemia market share @ Hypofibrinogenemia Market Report
Hypofibrinogenemia Overview
Hypofibrinogenemia is a rare bleeding disorder marked by low levels of fibrinogen, a vital protein essential for proper blood clotting. The condition may be either inherited genetically or acquired later in life. Fibrinogen plays a key role in the clotting process, and a deficiency can result in abnormal or excessive bleeding due to impaired clot formation. This disorder is diagnosed by specialized blood tests that measure fibrinogen levels and evaluate clotting function.
Hypofibrinogenemia causes include genetic mutations affecting fibrinogen production, liver disease impairing synthesis, and disseminated intravascular coagulation (DIC) leading to excessive consumption. Other causes of hypofibrinogenemia may involve severe infections, certain cancers, or medications that impact clotting factors.
Common hypofibrinogenemia symptoms include unusually heavy or prolonged bleeding after minor injuries, surgical procedures, or trauma to mucous membranes. Individuals may also bruise easily and suffer from frequent, extended nosebleeds. To diagnose hypofibrinogenemia, doctors typically perform detailed blood tests, including coagulation studies. If a hereditary form is suspected, genetic testing may be used to pinpoint mutations responsible for the deficiency.
Hypofibrinogenemia Epidemiology Segmentation
The hypofibrinogenemia epidemiology section provides insights into the historical and current hypofibrinogenemia patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The hypofibrinogenemia market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Diagnosed Prevalent Cases of Hypofibrinogenemia
- Type-specific Diagnosed Prevalent Cases of Hypofibrinogenemia
- Cause-specific Diagnosed Prevalent Cases of Acquired Hypofibrinogenemia
- Treatable Cases of Hypofibrinogenemia
Download the report to understand which factors are driving hypofibrinogenemia epidemiology trends @ Hypofibrinogenemia Epidemiological Insights
Hypofibrinogenemia Treatment Market
Current treatment approaches for hypofibrinogenemia generally focus on managing the root cause, such as liver disease or genetic disorders, while also aiming to increase fibrinogen levels to prevent excessive bleeding. In cases linked to liver dysfunction, liver transplantation may be considered a definitive solution. However, for most individuals, particularly those with congenital fibrinogen deficiency, ongoing care primarily involves fibrinogen replacement therapy.
Approved treatments for hypofibrinogenemia include fibrinogen replacement therapy using fibrinogen concentrate, cryoprecipitate, and plasma. Fibrinogen concentrate delivers a purified, standardized form of fibrinogen, allowing for accurate dosing and improved safety through viral inactivation procedures.
Cryoprecipitate, which contains fibrinogen, von Willebrand factor, and factor XIII, lacks viral inactivation steps, raising concerns about potential infections. Plasma is less commonly used due to risks of viral transmission and allergic reactions. In cases of dysfibrinogenemia, anticoagulants may be employed to mitigate the risk of thrombosis.
Commercial fibrinogen concentrates represent a dependable treatment option. These products are sourced from pooled donors and undergo extensive purification and viral inactivation to ensure safety. Compared to cryoprecipitate or fresh frozen plasma (FFP), fibrinogen concentrates offer enhanced viral safety and consistent potency, facilitating precise dosing.
While fibrinogen concentrates are the preferred choice when accessible, cryoprecipitate and FFP remain important alternatives when concentrates are unavailable. Products such as Clottafact (LFB), RiaStap (CSL Behring), and Fibryna (Octapharma) are effective fibrinogen concentrate therapies used in the treatment of hypofibrinogenemia.
Learn more about the hypofibrinogenemia treatment options @ Hypofibrinogenemia Treatment
Hypofibrinogenemia Emerging Drugs and Companies
Currently, only Biotest is working with its candidate BT-524 for hypofibrinogenemia treatment. BT-524 is a human fibrinogen concentrate derived from human plasma. Fibrinogen is a protein produced by the liver that plays a crucial role in blood clotting. A fibrinogen deficiency impairs the blood’s ability to clot, significantly increasing the risk of bleeding and delaying the cessation of bleeding. In congenital fibrinogen deficiency, patients either cannot produce fibrinogen or produce it in insufficient amounts.
In acquired fibrinogen deficiency, patients lose fibrinogen due to severe bleeding caused by trauma or surgery. In both situations, replenishing fibrinogen is necessary to stop the bleeding. The study aimed to assess the pharmacokinetics, effectiveness, and safety of BT-524 in 36 participants with specific conditions. BT-524 was administered intravenously, and its effects were observed over 14 days after a single dose.
The anticipated launch of this emerging therapy is poised to transform the hypofibrinogenemia market landscape in the coming years. As many of these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the hypofibrinogenemia market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about hypofibrinogenemia clinical trials, visit @ Hypofibrinogenemia Treatment Drugs
Hypofibrinogenemia Market Dynamics
The hypofibrinogenemia market dynamics are anticipated to change in the coming years. The hypofibrinogenemia market is primarily driven by the increasing prevalence of bleeding disorders, both congenital and acquired, which necessitate effective fibrinogen replacement therapies. Advances in diagnostic technologies have led to earlier and more accurate detection of fibrinogen deficiencies, spurring demand for treatment.
Additionally, growing awareness among clinicians and patients, coupled with rising healthcare expenditures and supportive reimbursement policies in developed markets, are further fueling market growth. The expansion of plasma-derived and recombinant fibrinogen products, along with ongoing research into novel therapies, also contributes significantly to market momentum. Moreover, regulatory incentives for orphan diseases are encouraging pharmaceutical companies to invest in treatment options for rare coagulation disorders like hypofibrinogenemia.
Furthermore, potential therapies are being investigated for the treatment of hypofibrinogenemia, and it is safe to predict that the treatment space will significantly impact the hypofibrinogenemia market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the hypofibrinogenemia market in the 7MM.
However, several factors may impede the growth of the hypofibrinogenemia market. A primary challenge is the rarity of the disorder, which leads to limited awareness among healthcare professionals and delayed or misdiagnosis in patients. Additionally, high costs associated with fibrinogen replacement therapies, such as cryoprecipitate and fibrinogen concentrates, pose a substantial financial burden, especially in low- and middle-income countries.
Regulatory hurdles also impact the timely approval and distribution of new therapies, further limiting treatment options. Moreover, the lack of large-scale clinical trials and real-world evidence restricts the ability to develop robust treatment guidelines and gain payer support. Collectively, these barriers contribute to an underserved patient population and stifle innovation in this niche therapeutic area.
Moreover, hypofibrinogenemia treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the hypofibrinogenemia market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the hypofibrinogenemia market growth.
| Hypofibrinogenemia Report Metrics | Details |
| Study Period | 2020–2034 |
| Hypofibrinogenemia Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Hypofibrinogenemia Market Size in 2023 | USD 200 Million |
| Key Hypofibrinogenemia Companies | CSL Behring, Octapharma, Biotest AG, and others |
| Key Hypofibrinogenemia Therapies | RiaSTAP, Fibryna, AdFIrst (BT-524), and others |
Scope of the Hypofibrinogenemia Market Report
- Hypofibrinogenemia Therapeutic Assessment: Hypofibrinogenemia current marketed and emerging therapies
- Hypofibrinogenemia Market Dynamics: Conjoint Analysis of Emerging Hypofibrinogenemia Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Hypofibrinogenemia Market Access and Reimbursement
Discover more about hypofibrinogenemia drugs in development @ Hypofibrinogenemia Clinical Trials
Table of Contents
| 1. | Hypofibrinogenemia Market Key Insights |
| 2. | Hypofibrinogenemia Market Report Introduction |
| 3. | Hypofibrinogenemia Market Overview at a Glance |
| 4. | Hypofibrinogenemia Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Hypofibrinogenemia Treatment and Management |
| 7. | Hypofibrinogenemia Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Hypofibrinogenemia Marketed Drugs |
| 10. | Hypofibrinogenemia Emerging Drugs |
| 11. | Seven Major Hypofibrinogenemia Market Analysis |
| 12. | Hypofibrinogenemia Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
Hypofibrinogenemia Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key hypofibrinogenemia companies, including Biotest AG, ICON PLC, Octapharma, CSL Behring, among others.
Hypofibrinogenemia Epidemiology Forecast
Hypofibrinogenemia Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted hypofibrinogenemia epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Hemophilia A Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key hemophilia A companies including Roche (Spark Therapeutics), ApcinteX, Alnylam Pharmaceuticals, Sanofi, Novo Nordisk A/S, Pfizer, Sangamo Therapeutics, Bayer, Ultragenyx Pharmaceutical, among others.
Hemophilia A Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key hemophilia A companies, including Takeda, Marinus Pharmaceuticals, SK biopharmaceuticals, CuroNZ, TAHO Pharmaceuticals, Axium Pharmaceuticals, among others.
Hemophilia Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key hemophilia companies, including ApcinteX, ASC Therapeutics, Ultragenix Pharmaceutical, BioMarin Pharmaceutical, CSL Behring, Freeline Therapeutics, Genentech, Inc., Novo Nordisk, Pfizer, Sanofi, Shire, Spark Therapeutics, Amarna therapeutics, Asklepios BioPharmaceutical, Bayer, Belief Biomed, Bioverativ, Catalyst Biosciences, Centessa Pharmaceuticals, Chameleon Biosciences, Chia Tai Tianqing Pharmaceutical Group, Expression Therapeutics, GC Pharma, GeneVentiv, Intellia tx, OPKO Health, Sangamo Therapeutics, Staidson Beijing BioPharmaceuticals, UBI Pharma, uniQure, among others.
Hemophilia B Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key hemophilia B companies, including ASC Therapeutics, Spark Therapeutics, Roche, Pfizer, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
